Electron configuration is a fundamental concept in chemistry and physics, detailing the distribution of electrons in the atomic orbitals of an atom or molecule. It is a representation of how electrons are distributed in various atomic and molecular orbitals, shells, and subshells.
The electron configuration for an element like bromine is crucial for understanding its chemical behavior, bonding patterns, and position in the periodic table. Electron configuration notation includes numbers, letters, and superscripts.
The numbers represent the energy levels, the letters (s, p, d, f) the type of orbital, and the superscripts are the number of electrons in those orbitals. For example, 1s² indicates the first energy level (1) with an s orbital containing two electrons.
The electrons fill up the orbitals following the Aufbau principle, Pauli exclusion principle, and Hund’s rule to achieve the most stable configuration.
Key Takeaways
- Bromine’s electron configuration is [Ar]3d¹⁰4s²4p⁵, indicating its placement in the periodic table as a halogen and its atomic structure.
- As a halogen, bromine is one electron short of a full octet, making it highly reactive and prone to forming compounds by gaining an electron.
- The filled 3d subshell of bromine affects its atomic size, ionization energy, and overall chemical behavior, despite not being in the outermost shell.
- Understanding bromine’s electron configuration is essential for predicting its bonding behavior, whether it forms ionic or covalent bonds.
- The principles of electron configuration, such as the Aufbau principle, Pauli exclusion principle, and Hund’s rule, are universal and applicable across different elements.
- Studying bromine’s electron configuration provides foundational knowledge for exploring and understanding the chemical behavior of other elements in the periodic table.
Bromine’s Position in the Periodic Table
Bromine is a halogen, situated in Group 17 and Period 4 of the periodic table. Its atomic number is 35, which means it has 35 protons and, in a neutral atom, 35 electrons.
The position of bromine in the periodic table gives the first hint towards its electron configuration, as elements in the same group share similar electron configurations in their outermost shell.
Electron Configuration
Bromine’s electron configuration can be written as [Ar]3d¹⁰4s²4p⁵. This notation indicates that bromine has the same electron configuration as argon (a noble gas) with additional electrons in the 3d, 4s, and 4p orbitals.
It has a total of 35 electrons, filling the orbitals in the order specified by the rules of electron configurations. The last electrons to be added are in the 4p orbital, reflecting its position in the p-block of the periodic table and its properties as a halogen.
Implications of Bromine’s Electron Configuration
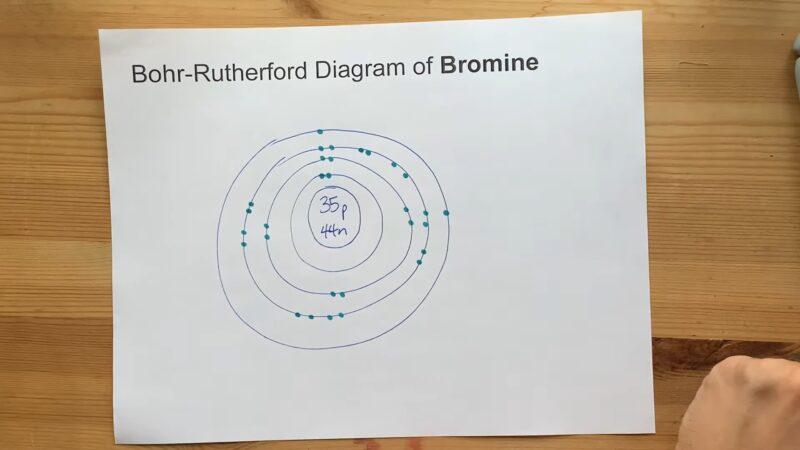
The electron configuration of bromine explains many of its chemical properties. With seven electrons in its outermost shell, bromine is one electron short of a full octet.
This makes it highly reactive, as it tends to accept an electron to achieve a stable noble gas configuration. This electron affinity is a hallmark of halogens.
Additionally, the filled 3d subshell contributes to the chemistry of bromine, affecting its atomic size, ionization energy, and ionic bonding patterns. Bromine’s electron configuration results in seven electrons in its outermost shell, making it particularly reactive.
This reactivity is due to its desire to complete an octet configuration, common among halogens. Bromine often participates in reactions that allow it to accept an electron, forming a bromide ion (Br-).
This electron affinity not only defines its reactivity but also its role in forming compounds, especially with metals where it can easily gain an electron to form ionic bonds. The filled 3d subshell of bromine has ten electrons which, although not in the outermost shell, significantly affect the element’s chemical behavior.
The 3d electrons add to the screening effect, shielding the outer electrons from the full charge of the nucleus. This results in a larger atomic radius compared to elements in the preceding period.
Furthermore, the filled 3d subshell contributes to the stabilization of the bromine atom, affecting its ionization energy and reactivity.
FAQs
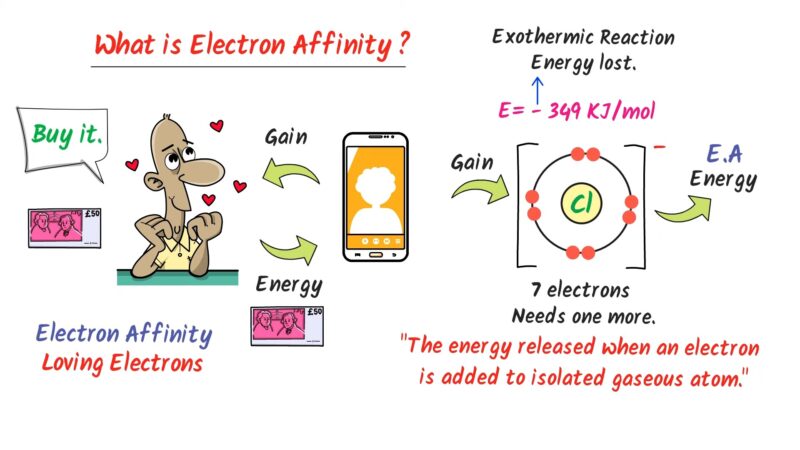
Why does bromine have a higher electron affinity compared to other elements?
Bromine has a higher electron affinity due to its position as a halogen, one electron short of a full octet. This makes it energetically favorable for bromine to gain an electron, completing its outer shell and achieving a stable noble gas configuration. Its atomic structure, with a nearly filled 4p subshell, creates a strong pull towards attracting that additional electron.
Can bromine ever lose electrons to form positive ions?
While less common than gaining an electron, bromine can lose electrons to form positive ions, particularly when interacting with elements much more electronegative than itself. This usually occurs in high-energy or highly reactive conditions, as removing an electron from bromine requires overcoming its electron affinity and ionization energy.
How does the electron configuration of bromine affect its placement in the periodic table?
Bromine’s electron configuration directly influences its placement in Group 17 (the halogens) and Period 4 of the periodic table. The configuration [Ar]3d¹⁰4s²4p⁵ demonstrates its shared properties with other halogens, all of which have seven electrons in their outermost shell, defining their reactivity and group characteristics.
Why is the 3d subshell filled before the 4s and 4p in bromine’s electron configuration?
According to the Aufbau principle, electrons fill lower energy orbitals first. The 3d orbitals have slightly lower energy than the 4s and 4p orbitals for elements in the fourth period of the periodic table, leading to the 3d orbitals being filled before the 4s and 4p in bromine’s electron configuration.
Does bromine form more stable compounds with metals or nonmetals?
Bromine generally forms more stable compounds with metals through ionic bonding, as it tends to gain an electron to form a bromide ion (Br-), which can then attract positively charged metal ions. However, it also forms stable covalent bonds with nonmetals by sharing electrons to achieve a full outer shell.
How does the electron configuration of bromine change when it forms a bromide ion?
When bromine forms a bromide ion (Br-), it gains one electron, filling its outermost p orbital and achieving a stable noble gas electron configuration similar to krypton. Its electron configuration changes from [Ar]3d¹⁰4s²4p⁵ to [Ar]3d¹⁰4s²4p⁶, indicating a complete octet in the outermost shell.
Final Words
Understanding the electron configuration of bromine involves more than just memorizing the arrangement of electrons. It requires a fundamental understanding of atomic orbitals, principles of electron filling, and the implications of this configuration on chemical behavior.
Bromine’s electron configuration ([Ar]3d¹⁰4s²4p⁵) is a testament to its reactivity and position as a halogen. This configuration affects everything from the atom’s size and energy to its reactivity and bonding characteristics, showcasing the profound impact that electron configuration has on the chemical identity of an element.

