Welcome to a comprehensive exploration of the carbon dioxide molecule or CO2. It’s more than just gas; it’s a topic brimming with intriguing concepts and fascinating scientific facts.
Let’s dive in to explore the Lewis structure, molecular geometry, and hybridization of CO2 and see what makes this molecule so remarkable.
The Basics of Lewis Structures
Lewis structures, also known as electron dot diagrams, were introduced by Gilbert N. Lewis in 1916. They play a crucial role in visualizing the arrangement of valence electrons among atoms in a molecule, helping us predict its physical and chemical properties.
A Lewis structure is a type of shorthand notation that scientists use to describe the distribution of electrons in molecules. By learning to read these diagrams, you can gain a deeper understanding of the bonds that hold atoms together and the lone pairs of electrons that can affect a molecule’s behavior.
- Atoms: The atoms in a molecule are represented by their chemical symbols.
- Bonds: The bonds between atoms are depicted as lines. A single line represents a single bond (two electrons), a double line a double bond (four electrons), and a triple line a triple bond (six electrons).
- Lone pairs: Lone pairs, or non-bonding pairs of electrons, are shown as dots. They can affect a molecule’s shape and reactivity.
Lewis structures are a foundational tool in chemistry, allowing us to visualize how atoms share or transfer electrons to form molecules.
Lewis Structure of CO2
When creating the Lewis structure for carbon dioxide, we start with the central carbon atom, which forms double bonds with two oxygen atoms on either side. This process highlights the importance of understanding valence electrons as they determine how each atom interacts and forms bonds.
- Valence electrons: Carbon has four valence electrons, while each oxygen atom has six. The total number of valence electrons in a CO2 molecule is, therefore 4 + 2(6) = 16.
- Formation of bonds: Carbon forms double bonds with each oxygen atom, each bond consisting of two shared electron pairs.
- Resulting structure: The resulting Lewis structure of CO2 shows a carbon atom at the center with double bonds to two oxygen atoms, with each oxygen atom having two lone pairs.
Exploring Molecular Geometry
Molecular geometry is the three-dimensional arrangement of atoms in a molecule. It gives insight into the molecule’s shape, which is essential in determining its polarity, reactivity, phase of matter, color, magnetism, biological activity, etc.
VSEPR Theory and Molecular Geometry
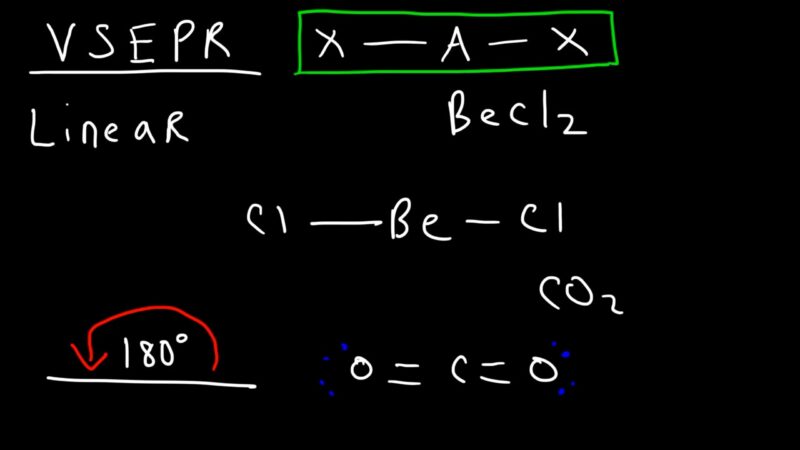
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. According to the VSEPR theory, electron pairs will arrange themselves to minimize repulsion, which results in a specific geometric shape for the molecule.
- Linear: Two regions of electron density will arrange themselves linearly.
- Trigonal planar: Three regions of electron density will form a flat, triangular shape.
- Tetrahedral: Four regions of electron density will arrange themselves in a tetrahedron.
CO2 Molecular Geometry
Applying the VSEPR theory to CO2, we can predict its molecular geometry. The carbon atom in CO2 has two electron groups; both shared with the oxygen atoms through double bonds. There are no lone pairs of electrons around the central carbon atom.
Therefore, the two electron groups will arrange themselves in a straight line, resulting in a linear molecular geometry for CO2. The bond angle is 180°.
The Concept of Hybridization
Hybridization is a theoretical concept in chemistry that describes the rearrangement of atomic orbitals in atoms with multiple bonds. This process results in hybrid orbitals, which have different shapes and energies than the original atomic orbitals.
How It Works
Hybridization can be visualized as a blending of atomic orbitals within an atom. It allows for the explanation of molecule shapes that cannot be described by the valence bond theory alone. There are several types of hybridization, including:
- sp: One s orbital and one p orbital combine to form two sp hybrid orbitals.
- sp2: One s orbital and two p orbitals mix to form three sp2 hybrid orbitals.
- sp3: One s orbital and three p orbitals merge, resulting in four sp3 hybrid orbitals.
The type of hybridization occurring in an atom can affect the shape of the molecules it forms and its chemical reactivity.
CO2 Hybridization
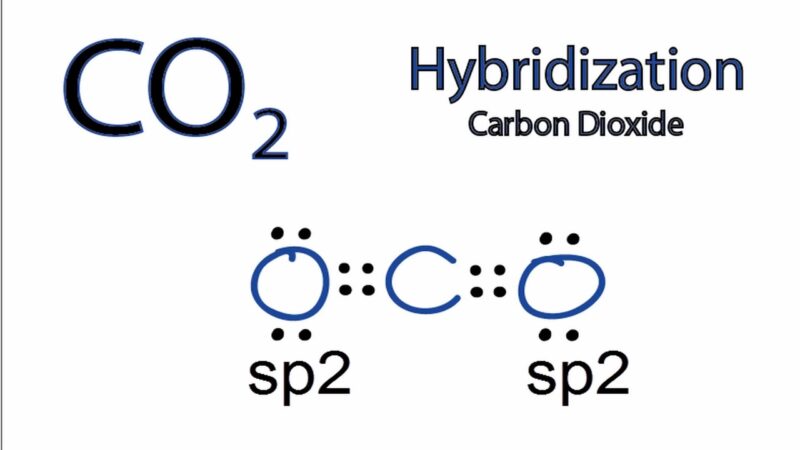
In the case of CO2, the carbon atom undergoes sp hybridization. This occurs because the carbon atom has two regions of electron density (from the two double bonds with oxygen), and these form two sp hybrid orbitals. The oxygen atoms retain their unhybridized p orbitals, which overlap with the carbon’s sp hybrid orbitals to form π bonds. This results in a molecule with a linear shape.
The Role of Electronegativity in Molecular Structures
Electronegativity is a measure of how strongly an atom attracts electrons in a bond. It’s a critical factor in determining the type of bond formed between atoms and the molecule’s overall polarity.
Electronegativity
Electronegativity values are on a scale, with fluorine (the most electronegative element) at the top with a value of 4.0. This concept helps to determine if a bond is covalent (where electrons are shared between atoms) or ionic (where electrons are transferred from one atom to another).
- Low Electronegativity Difference: If the electronegativity difference between two atoms is small (usually less than 1.7), the bond is likely to be covalent.
- High Electronegativity Difference: If the electronegativity difference is large (usually greater than 1.7), the bond is likely to be ionic.
Understanding electronegativity is essential for predicting how different atoms will interact with each other, leading to the formation of various molecular structures.
CO2 and Electronegativity
In carbon dioxide, both the carbon and oxygen atoms share electrons, indicating that the bonds are covalent. But are they nonpolar covalent or polar covalent? To answer this question, we need to consider both electronegativity and molecular geometry.
- Electronegativity values: Carbon has an electronegativity value of 2.55, and oxygen has a value of 3.44.
- Difference: The difference in electronegativity values between carbon and oxygen is 3.44 – 2.55 = 0.89, which is less than 1.7, indicating a polar covalent bond.
However, due to the linear geometry of the CO2 molecule, the dipole moments of the two C=O bonds cancel each other out, making the overall molecule nonpolar.
Polar vs Nonpolar Molecules
The polarity of a molecule has significant effects on its physical and chemical properties, like boiling point, solubility, and reactivity. Understanding the difference between polar and nonpolar molecules is a crucial aspect of chemistry.
Polar Molecules
Polar molecules have an uneven distribution of electron density, leading to a positive charge on one side and a negative charge on the other. This polarity is caused by a significant difference in electronegativity between the atoms in the molecule and a molecular structure that doesn’t cancel out the individual bond polarities.
Polar molecules often have higher boiling points due to the strong dipole-dipole interactions between the molecules.
Nonpolar Molecules
Nonpolar molecules have a uniform distribution of electron density, which means there is no overall positive or negative charge across the molecule. This can occur when the molecule consists of identical atoms (like O2 or N2) or when the molecule’s geometry leads to a cancellation of polar bonds, as in CO2.
Nonpolar molecules generally have lower boiling points due to weaker van der Waals forces between the molecules. In our study molecule, CO2, while the C=O bonds are polar due to a difference in electronegativity, the linear geometry of the molecule results in the overall molecule being nonpolar.
FAQs
How is the Lewis structure of CO2 drawn?
The Lewis structure of CO2 is drawn by first placing the carbon atom in the center as it is less electronegative. Then, two double bonds are drawn between the carbon and each oxygen atom, representing the sharing of four electrons (two from carbon and two from each oxygen).
What is hybridization in CO2?
In CO2, the carbon atom undergoes sp hybridization. This type of hybridization occurs as a result of carbon being bound to two other atoms. The carbon atom uses one s and one p orbital to form two sp hybrid orbitals, which are used to form sigma bonds with the oxygen atoms.
What is the hybridization of the oxygen atoms in CO2?
In CO2, each oxygen atom hybridizes its orbitals to form three sp2 hybrid orbitals. One of these sp2 orbitals is used to form a sigma bond with the carbon atom, and the remaining p orbital is used to form a pi bond.
What is the molecular geometry of CO2?
The molecular geometry of CO2 is linear. This is due to the presence of two sigma bonds and the repulsion of the electron pairs, which forces them to move to the opposite sides of the carbon atom.
What is the bond angle in CO2?
The bond angle in CO2 is 180 degrees, which is characteristic of molecules with a linear shape.
Why is the CO2 molecule linear?
The CO2 molecule is linear because of the symmetric distribution of the atoms and the repulsion between the electron pairs in the double bonds. This repulsion forces the atoms to arrange themselves as far apart as possible, resulting in a linear shape.
What is the significance of the Lewis structure and hybridization of CO2?
The Lewis structure and hybridization of CO2 help us understand how the atoms and electrons are arranged in the molecule. This can help us determine the molecular geometry, how the molecule might react with other molecules, and some of the physical properties of the molecule.
Are there any limitations to the Lewis structure model?
Yes, while Lewis structures capture many of the key features of the electronic structure of a range of molecular systems, there are simple and archetypal molecular systems for which a Lewis description is misleading or inaccurate.
For instance, Lewis structures do not offer an explanation for why cyclic C6H6 (benzene) experiences special stabilization beyond normal delocalization effects, while C4H4 (cyclobutadiene) actually experiences a special destabilization. Molecular orbital theory provides the most straightforward explanation for these phenomena.
Final Words
The Lewis structure, molecular geometry, and hybridization of the CO2 molecule provide a wealth of information about its properties. This simple molecule offers a useful study case for these concepts, allowing students and chemistry enthusiasts to better understand and predict the behavior of molecules.