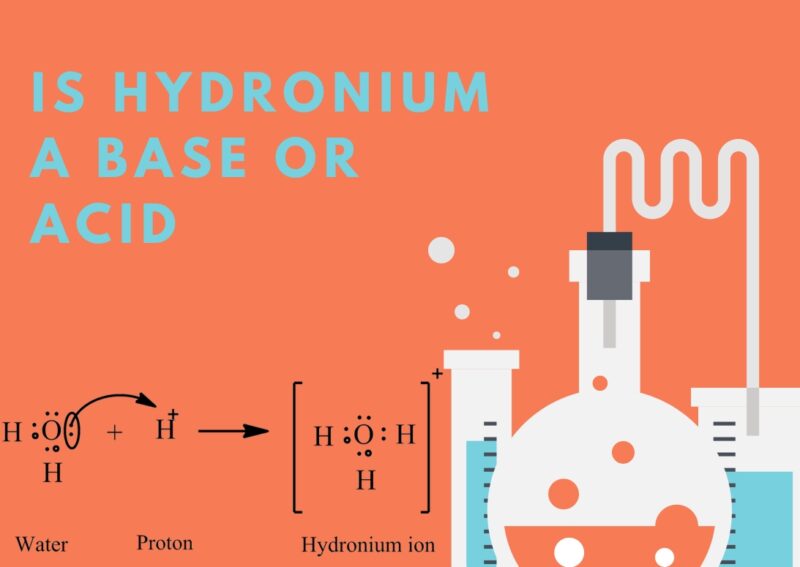
Today we explore the hydronium ion (H3O+). Comprising a water molecule (H2O) that has enthusiastically embraced an extra proton (H+), thus adopting a positive charge.
Hydronium ions do not shy away from involvement; they play pivotal roles in an extensive range of chemical reactions, especially in aqueous environments. However, their very existence poses a captivating puzzle: Should we classify it as a base or an acid?
To untangle this quandary, it becomes necessary to step onto the foundational bedrock of acid-base chemistry and embark on a detailed exploration.
Acids vs Bases
First up, it is essential to grasp the underlying principles of acids and bases with three key definitions have illuminated our understanding.
- The Arrhenius definition propounds that an acid, when submerged in water, generates H+ ions. In contrast, a base, in similar conditions, produces OH- ions.
- The Bronsted-Lowry definition designates acids as substances eager to donate protons, while bases stand ready to accept them.
- The third and final interpretation comes from Gilbert N. Lewis. Lewis defines acids as electron pair acceptors and bases as electron pair donors
Properties
Hydronium ions manifest in aqueous solutions when a water molecule acquiesces to the addition of an extra proton from another water molecule, leading to the birth of a hydronium ion and a hydroxide ion (OH-).
Water
Water is not a silent partner in this process. Its polar nature, signified by a distinct positive end (hydrogen) and a negative end (oxygen), fosters the ideal conditions for the interaction and transfer of protons. This unique attribute of water opens the doors to the formation of hydronium ions.
Charge
Beyond its formation, the ion is characterized by its positive charge and its readiness to partake in proton exchange reactions, courtesy of its extra proton. Additionally, it is a central player in the auto-ionization of water and sets the stage for a more nuanced discussion on its potential identity as an acid or a base.
As an Acid
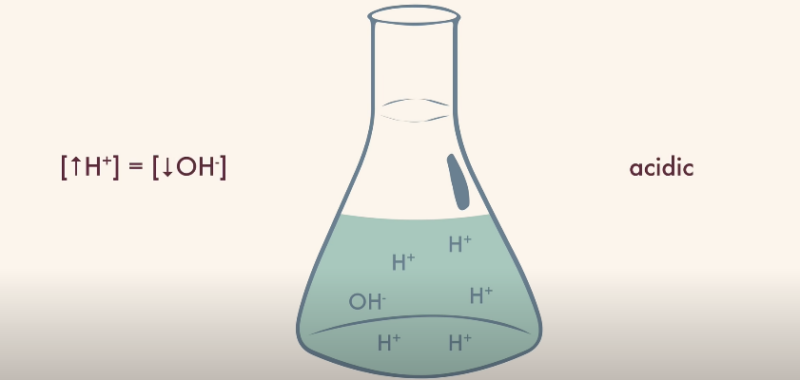
The first perspective we adopt is that of the Arrhenius definition. Since an acid is something that increases the concentration of H+ ions in an aqueous solution, hydronium fits snugly into this definition. It carries an extra proton and can donate it readily, therefore behaving as an acid.
From the Bronsted-Lowry viewpoint, hydronium still emerges as an acid, where acids are proton donors. Hydronium, in a solution, can relinquish its additional proton, thereby fulfilling the characteristics of a Bronsted-Lowry acid.
Even within the broader frame of the Lewis definition, it does not stray from the acid territory. Since Lewis acids accept electron pairs, and the additional proton is more than eager to do so, we can confidently categorize hydronium as an acid by the Lewis definition as well.
As a Base
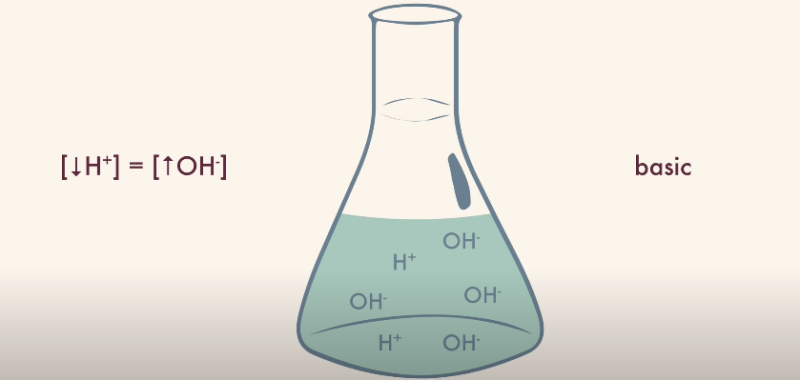
The argument for hydronium as a base might seem counterintuitive. When viewed in the context of certain chemical reactions, it can be seen as accepting a proton rather than donating one. This act of proton acceptance aligns with the behavior of a base as per the Bronsted-Lowry definition.
Further evidence comes from its role in the formation of the ion itself. In this process, a water molecule acts as a base, accepting a proton to become a hydronium ion.
However, counterarguments challenge this perspective, primarily by highlighting that the basic action is more accurately ascribed to the water molecule and not the resulting hydronium ion.
The Autoionization of Water
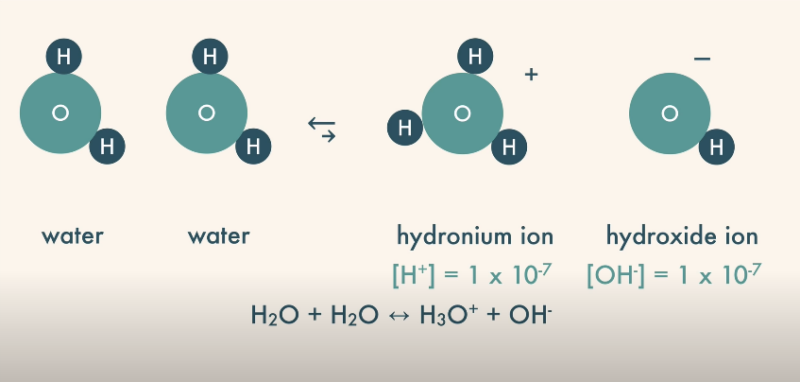
The self-ionization (or autoionization) of water is a critical process that beautifully illustrates the intricate dance between acids and bases in aqueous solutions.
In this spontaneous reaction, two water molecules interact, with one acting as a Bronsted-Lowry acid, donating a proton, and the other as a Bronsted-Lowry base, accepting the proton. The outcome of this interaction is the formation of hydronium and hydroxide ions.
The concentration of hydronium ions and hydroxide ions in pure water at room temperature is constant, and their product is known as the ion product of water, a key concept in understanding the pH scale. The pH scale, which measures the acidity or basicity of a solution, hinges on the concentration of hydronium ions, underscoring their importance in the acid-base realm.
Experimental Evidence
Concrete evidence supporting dualistic behavior has been provided by numerous scientific studies and experiments. Research has shown they can act as both proton donors and acceptors, substantiating the theories discussed above.
- In one instance, hydronium ions were observed to donate a proton to ammonia, a weak base, substantiating their role as acids.
- In another scenario, hydronium ions were seen to accept a proton from a strong acid, such as hydrochloric acid, echoing the behavior of a base.
These experiments highlight the context-dependent behavior of hydronium ions.
Real-World Applications
Industrial processes such as acid-base titrations, pH adjustments in food and beverage industries, and chemical synthesis all rely heavily on understanding the behavior of hydronium ions.
Beyond industry, many biochemical reactions in our bodies are acid-base reactions, and the pH of our blood—a direct measure of hydronium ion concentration—is tightly regulated to maintain optimal body function.
In the environmental arena, it determines the acidity of rainwater and bodies of water, influencing aquatic ecosystems’ health. Hence, understanding nature is not just a matter of academic interest but of practical, real-world consequence.
Fun Facts
- The name “hydronium” is derived from “hydro-” (Greek for water) and “-onium” (used to denote ions).
- The hydronium ion is smaller than a water molecule, even though it’s made of a water molecule plus an extra proton.
- Hydronium ions are crucial in the food industry; they’re used in the production of certain types of jellies and candies.
- The concentration of hydronium ions in pure water is only about 1 in 10 million molecules.
- The extra proton in a hydronium ion is not fixed to one of the water molecule’s hydrogens; instead, it zips around all three, forming a sort of “cloud” of charge.
FAQ
What gives hydronium ions their positive charge?
Comes from the extra proton that a water molecule has accepted.
Can there be hydronium ions in a non-aqueous solution?
They can exist in non-aqueous solutions. However, their concentration and behavior may differ compared to aqueous solutions.
How does temperature affect hydronium ion concentration?
Higher temperatures can increase ionization and concentration.
What happens when hydronium concentration is increased in a solution?
Makes the solution more acidic, resulting in a lower pH.
Can hydronium ions exist outside of a solution, such as in a gas form?
They can indeed exist in gaseous form and have been detected in interstellar space.
Conclusion
After this thorough examination of the hydronium ion, its character, and its roles, we revisit the initial question with a more enlightened perspective: Is it a base or an acid? The answer, as we’ve uncovered, is not a simple dichotomy but a reflection of the multifaceted nature of hydronium.
They can act as both an acid and a base, depending on the context and the chemical reaction they are involved in.
- We’ve discussed the various definitions of acids and bases, each offering its own lens to examine hydronium.
- We’ve established that hydronium typically behaves as an acid but can also exhibit basic properties under certain conditions.
- We explored the vital role of hydronium in the autoionization of water, proton transfer reactions, and the establishment of acid-base equilibria.
- Real-world applications and experimental evidence further underscore the importance of understanding the complex nature of hydronium ions.
As for the future of research, it promises to continue to be a topic of fascination. The story of the hydronium ion is far from over, and I, for one, eagerly await the next chapter.