Aniline, an organic compound with the formula C6H5NH2, is a fascinating substance that plays a significant role in various industries. This simple aromatic amine is a colorless liquid with a characteristic odor.
It’s slightly soluble in water, making it a versatile substance in various chemical reactions. Its unique properties have made it a vital component in many industrial processes, from dye manufacturing to pharmaceuticals .
Main Characteristics
Physical Properties
Its physical properties make it a versatile substance in various industrial applications. It’s a colorless to slightly yellow liquid with a characteristic odor. It has a boiling point of 184°C and a melting point of -6°C. These properties make it a versatile substance in various industrial applications.
It’s slightly soluble in water but readily soluble in most organic solvents, which makes it a useful solvent in its own right and a key ingredient in many chemical reactions.
Chemical Properties
Chemically, aniline is a weak base and can form salts with acids. This property is crucial in its use in many industrial processes. It’s also a nucleophile, meaning it can donate a pair of electrons to form a new bond.
This property makes this substance a key player in many chemical reactions, particularly in the synthesis of dyes and drugs. Its ability to form new bonds makes it a versatile building block in the world of chemistry.
Production
Aniline is primarily produced industrially through a two-step process that involves the nitration of benzene followed by the reduction of the resulting nitrobenzene. This process is a testament to the power of chemical reactions to transform simple substances into more complex ones.
Nitration of Benzene
The first step, nitration, involves the reaction of benzene with nitric acid and sulfuric acid. This process forms nitrobenzene. The reaction is exothermic, meaning it releases heat. The nitro group (-NO2) replaces one of the hydrogen atoms on the benzene ring.
This process is a carefully controlled one, as the reaction mixture is kept below 50°C to prevent the formation of dinitrobenzene, a byproduct that forms at higher temperatures.
Reduction of Nitrobenzene
The reduction of nitrobenzene is the second step in the production of aniline. This process involves the reaction of nitrobenzene with hydrogen in the presence of a metal catalyst, such as iron or nickel. The nitro group (-NO2) on the nitrobenzene is reduced to an amino group (-NH2), forming the substance.
This reaction is also exothermic and is carried out under high pressure and temperature. This process showcases the transformative power of chemical reactions, turning a relatively simple molecule into a more complex one with a wide range of uses.
Uses of Aniline

This substance has a wide range of uses, thanks to its chemical properties. It’s primarily used as a precursor to many chemicals, including dyes, drugs, and plastics.
Plastics Manufacturing
Its most significant use is in the manufacture of methylene diphenyl diisocyanate (MDI), a precursor to polyurethane plastics. These plastics are used in a variety of applications, from insulation and furniture to automotive parts. This wide range of applications showcases the versatility of aniline and its importance in modern industry.
Dye Manufacturing
Aniline is a key ingredient in the production of many dyes. The first synthetic dye, mauveine, was made from the substance by Sir William Henry Perkin in 1856. This discovery marked the beginning of the synthetic dye industry, which has since grown to become a major global industry.
Today, aniline dyes are used in a variety of applications, including textiles, leather, and paper. They’re known for their bright colors and excellent colorfastness, making them a popular choice for many applications.
Drug Synthesis
Itis also used in the synthesis of certain pharmaceuticals. Its nucleophilic properties make it a useful building block in the production of various drugs, including paracetamol (acetaminophen), a common over-the-counter pain reliever and fever reducer.
In addition to paracetamol, aniline is used in the synthesis of other drugs, such as sulfanilamide, a sulfa drug used to treat bacterial infections. This use of the substance showcases its importance in the world of medicine, where it helps to alleviate pain and fight infections.
Health and Environmental Implications
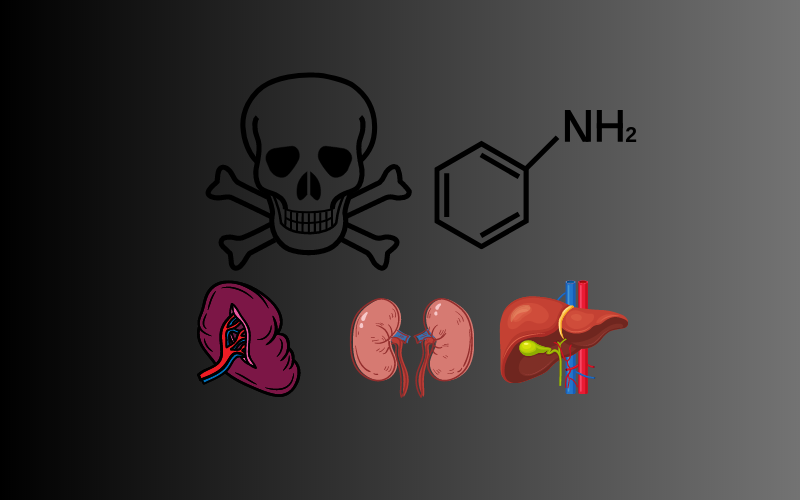
While aniline is a valuable industrial chemical, it’s not without its health and environmental implications. Exposure to this substance can have harmful effects on human health, and it can also impact the environment.
Health Effects
This substance is toxic if ingested, inhaled, or absorbed through the skin. It can cause symptoms such as headaches, dizziness, and blue-colored skin. Long-term exposure can lead to more serious health effects, including damage to the spleen, liver, and kidneys.
Acute exposure to aniline can cause a variety of symptoms, including headaches, dizziness, and methemoglobinemia, a condition characterized by a reduced ability of the blood to carry oxygen, leading to blue-colored skin and lips. This condition, also known as “blue baby syndrome,” can be life-threatening in severe cases.
Chronic Exposure and Long-Term Effects
Chronic exposure to the substance can lead to more serious health effects. These include damage to the spleen, liver, and kidneys, as well as an increased risk of bladder cancer. Therefore, it’s essential to handle aniline with care and use appropriate protective equipment.
Environmental Impact
Aniline can also have a significant impact on the environment. It’s moderately toxic to aquatic life and can contaminate water sources if not properly managed. In the environment, this substance can undergo biological degradation, but this process can be slow, leading to its accumulation.
Therefore, it’s crucial to prevent spills and to treat industrial wastewater containing the substance before its release into the environment. This environmental impact underscores the importance of responsible handling and disposal of aniline.
Safety Precautions

Aniline is a toxic and potentially hazardous chemical compound, and working with it requires strict safety precautions to protect yourself and others. Here are some essential safety measures to consider when handling this substance:
Personal Protective Equipment (PPE)
Wear appropriate protective clothing, such as lab coats, long-sleeved shirts, and long pants, to minimize skin exposure. Use chemical-resistant gloves (e.g., nitrile or neoprene) to prevent skin contact.
Wear safety goggles or a full-face shield to protect your eyes from splashes or fumes. Use a chemical-resistant apron or smock for additional protection.
Ventilation
Work in a well-ventilated area to reduce exposure to aniline vapors. If possible, use a fume hood or local exhaust ventilation system. Avoid working with it in confined spaces where vapors can accumulate.
Handling and Storage
Store aniline in a secure, well-labeled container, away from incompatible substances. Keep the container tightly closed when not in use. Do not store or handle this substance near open flames or ignition sources.
Spill and Leak Procedures

Have a spill kit readily available and know the proper procedures for cleaning up small spills. In case of a large spill or leak, evacuate the area, and seek assistance from trained personnel.
Avoid Ingestion and Inhalation
Never eat, drink, or smoke in areas where aniline is used or stored. Avoid breathing in vapors or dust. Use respiratory protection if necessary.
First Aid
Familiarize yourself with the potential health hazards of aniline and know the appropriate first aid measures in case of exposure.
Emergency Preparedness
Have access to emergency showers and eyewash stations in case of accidental exposure to aniline.
Training and Knowledge
Make sure all personnel handling aniline are adequately trained in its safe handling and storage. Be aware of the Material Safety Data Sheet (MSDS) or Safety Data Sheet (SDS) for the substance and follow the recommended safety guidelines.
Waste Disposal

Dispose of aniline and its waste products according to local regulations and in compliance with proper waste disposal procedures for hazardous chemicals. Medical Monitoring: Consider implementing a medical monitoring program for individuals who regularly work with this substance to detect any potential health effects early on.
Remember that this is a highly toxic substance, and even low levels of exposure can have adverse health effects. Always prioritize safety and take the necessary precautions to protect yourself and those around you when working with aniline.
FAQs:
Can aniline be detected in the environment?
Yes, it can be detected and quantified in environmental samples using analytical techniques like gas chromatography-mass spectrometry (GC-MS).
Does it react with other chemicals?
Yes, aniline can undergo various chemical reactions, including acylation, alkylation, and diazotization.
How is it used in the production of polyurethane foams?
This substance is an important intermediate in the production of polyurethane foams, which are used in various applications, including furniture and insulation.
Can it be used as a solvent?
Yes, it can be used as a solvent for various substances, including oils, resins, and waxes.
Are there any regulations governing its use?
Yes, various regulatory bodies impose restrictions on aniline use to protect human health and the environment.
Conclusion
Aniline is a versatile compound with a wide range of uses, from dye manufacturing to drug synthesis. However, its use comes with health and environmental implications that need to be carefully managed.
As we continue to rely on this substance for various applications, it’s crucial to understand its properties, uses, and impacts to ensure its safe and sustainable use. That way we can continue to benefit from its many uses while minimizing its potential harm.

