Rubidium is an intriguing element found in the periodic table, characterized by its unique chemical and physical properties. To understand the essence of Rubidium, particularly its valence electrons, it’s crucial to explore various aspects of this element, from its position in the periodic table to its electron configuration and its role in various applications.
Rubidium in the Periodic Table
The periodic table is a systematic arrangement of elements based on their atomic numbers and electronic configurations. Rubidium, with the atomic symbol ‘Rb’ and atomic number 37, falls into the category of alkali metals.
This group is known for its reactivity and distinctive properties. Rubidium, located in the fifth period and the first group of the periodic table, shares common traits with other alkali metals like Lithium (Li), Sodium (Na), Potassium (K), and Cesium (Cs).
Electron Configuration of Rubidium
The electron configuration of an element is a representation of how electrons are distributed in its atomic orbitals. For Rubidium, the electron configuration can be represented as [Kr]5s¹.
This configuration indicates that Rubidium has electrons filling up to the 5th energy level. The presence of one electron in its outermost s-orbital (5s¹) is particularly significant in understanding its chemical behavior.
Valence Electrons and Their Significance
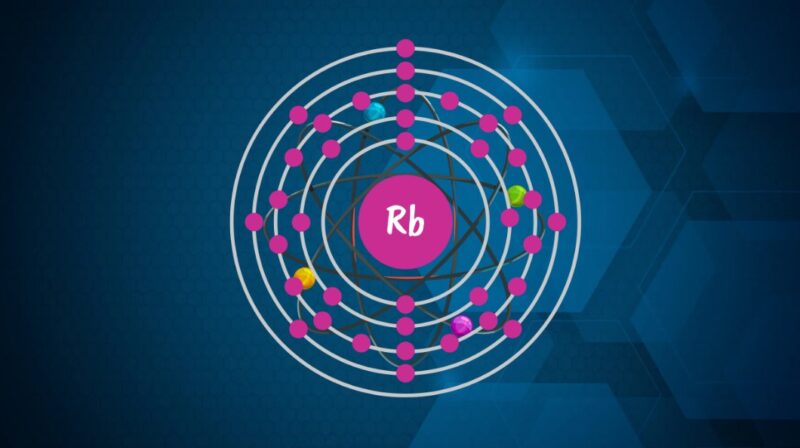
Valence electrons are the electrons located in the outermost shell of an atom. They play a crucial role in chemical bonding and reactions. In the case of Rubidium, the single electron in the 5s orbital is its valence electron. This electron largely determines how Rubidium reacts chemically and its placement in the periodic table.
Chemical Properties of Rubidium
Rubidium, like other alkali metals, exhibits highly reactive behavior. This reactivity is attributed to its single valence electron, which it can easily lose to attain a more stable electronic configuration. Upon losing this electron, Rubidium forms a +1 ion (Rb⁺). This ion formation underlies many of its chemical reactions and compounds.
Rubidium in Chemical Compounds
Rubidium forms various compounds, primarily through ionic bonding. In these compounds, Rubidium typically exhibits a +1 oxidation state, aligning with its tendency to lose one electron.
Compounds such as Rubidium chloride (RbCl) and Rubidium hydroxide (RbOH) are common examples, showcasing how Rubidium’s valence electron participates in chemical bonding.
Applications of Rubidium
Rubidium’s unique properties, influenced by its valence electron, make it suitable for various applications. It’s used in electronics, specialized glasses, and even as a working fluid in vapor turbines. Additionally, its isotopes find applications in medical imaging and research.


